|
|
Devin B. Lowe, Ph.D.
Associate Professor
Department of Immunotherapeutics and Biotechnology
TTUHSC School of Pharmacy
1718 Pine Street
Office - Room 1306
Lab - Room 1315
Abilene, TX 79601
Tel: 325-696-0486
E-mail: devin.lowe@ttuhsc.edu
RESEARCH FOCUS
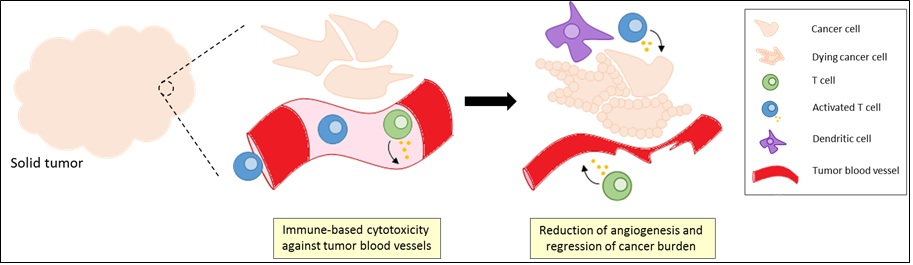
Our work centers on the fascinating areas of tumor immunology and tumor angiogenesis.
There is no question the power of the immune system in preventing or resolving cancer. Decades of pre-clinical work has now matured into viable anti-cancer therapeutics that are showing significant survival benefits in patients. One has to look only as far as gene-engineered effector T cells or specific antibody-based therapies to appreciate the power of the immune system in halting tumorigenesis.
However, we are also readily aware of the immuno-suppressive/-evasive nature of the solid tumor microenvironment (TME) that severely diminishes the effectiveness of antibodies and T cells. Established vascularized cancers tend to contain mature and immature vessels that confound blood flow rates within the lesion and promote localized areas of hypoxia and acidosis. The tumor-influenced endothelium also downregulates activating adhesion molecules that ultimately abrogate a T cells functional ability to traffick to and localize within the tumor bed. Yet, in cases where the effector T cell gains access to tumor cells, a layered fortress of immunosuppression awaits. TME-residing cells such as regulatory T cells and myeloid-derived suppressor cells elaborate soluble molecules that strip away a T cells ability to fight. Immune checkpoints have also received a great deal of attention and involve the TME engaging certain T cell molecules to downmodulate any forthcoming immune response.
So, quite frankly, as the field moves forward to design and implement highly potent cancer immunotherapies, durable clinical responses will be unattainable unless decisive action is also taken against many of the TME-derived properties mentioned above.
To this end, there are two interrelated areas in the lab that receive our focus:
- At the basic science level, we are performing studies to understand the mechanistic aspects and consequences of destroying components of tumor-derived blood vessels using the immune system. Additionally, we are attempting to improve our knowledge of the interplay between TME components such as immune cells, cancer cells, and the vasculature.
- At the translational science level, we are developing immunotherapeutic strategies that target tumor angiogenesis with the goal of downstream testing in prospective clinical trials. We are also creating diagnostic assays to help inform our immunotherapeutic designs and implementation in patients with cancer.
Select publications:
Wooster AL, Anderson TS, Lowe DB. Expression and characterization of soluble epitope-defined major histocompatiblity complex (MHC) from stable eukaryotic cell lines. J Immunol Methods, In press, 2018.
Lowe DB, Bivens CK, Mobley AS, Herrera CE, McCormick AL, Wichner T, Sabnani MK, Wood LM, Weidanz JA. TCR-like antibody drug conjugates mediate killing of tumor cells with low peptide/HLA targets. mAbs 2017 May/Jun;9(4):603-614.
Lowe DB, Bose A, Taylor JL, Tawbi H, Lin Y, Kirkwood JM, Storkus WJ. Dasatinib promotes the expansion of a therapeutically superior T-cell repertoire in response to dendritic cell vaccination against melanoma. Oncoimmunology 2014 Jan 1;3(1):e27589.
Qu Y, Chen L, Lowe DB, Storkus WJ, Taylor JL. Combined Tbet and IL12 gene therapy elicits and recruits superior antitumor immunity in vivo. Mol Ther 2012 Mar;20(3):644-51.
Zhao X, Bose A, Komita H, Taylor JL, Chi N, Lowe DB, Okada H, Cao Y, Mukhopadhyay D, Cohen PA, Storkus WJ. Vaccines targeting tumor blood vessel antigens promote CD8(+) T cell-dependent tumor eradication or dormancy in HLA-A2 transgenic mice. J Immunol 2012 Feb 15;188(4):1782-8.
Zhao X, Bose A, Komita H, Taylor JL, Kawabe M, Chi N, Spokas L, Lowe DB, Goldbach C, Alber S, Watkins SC, Butterfield LH, Kalinski P, Kirkwood JM, Storkus WJ. Intratumoral IL-12 gene therapy results in the crosspriming of Tc1 cells reactive against tumor-associated stromal antigens. Mol Ther 2011 Apr;19(4):805-14.
Patent:
Storkus WJ, Bose A, Taylor JL, Zhao X, Lowe DB. Immunogenic tumor associated stromal cell antigen peptides and methods of their use. United States Patent # 9,345,770.
We are grateful to our funding sources: NIH (R15 CA215874), DOD (W81XWH-18-1-0293), and Dodge Jones Foundation
|